
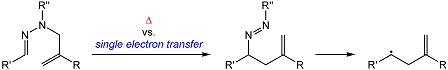
N-Allylhydrazones are reported to undergo an elaborate [3,3]-sigmatropic shift/nitrogen extrusion sequence. We have investigated both concerted and radical cation pathways for the [3,3]-sigmatropic shift of several N-allylhydrazones using density functional calculations. It was discovered that, assuming facile formation of an N-allylhydrazone radical cation, the rearrangement takes place through a series of transformations with a much lower barrier than the 6-electron concerted alternative that is available to the neutral N-allylhydrazone. Subsequent nitrogen extrusion to form the corresponding homoallyl radical was also found to be extremely facile.
Siebert, M. R.; Tantillo, D. J. Org. Lett. 2008, 10, 3219-3222: "[3,3]-Sigmatropic Shifts of N-Allylhydrazones: Quantum Chemical Comparison of Concerted and Radical Cation Pathways"
Osvaldo Gutierrez, Regan J. Thomson and Dean J. Tantillo: "Origin of Reactivity in the Carbon-Carbon 'Traceless Bond Construction' Process via a Triflimide-Catalyzed Sigmatropic Rearrangment of N-Allylhydrazones." Poster presented by Osvaldo Gutierrez at the R. Bryan Miller Symposium, UC Davis, Davis, CA, April 8-9, 2010.