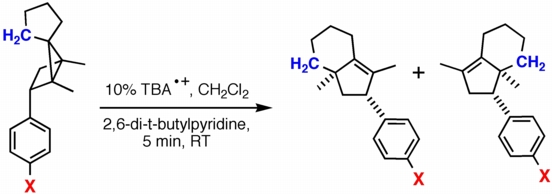
In collaboration with Dan Little at UC, Santa Barbara, we are exploring radical cation rearrangements such as the reaction shown above. In these reactions, an electron is first removed from the strained sigma-bond of the [2.1.0] ring system (known as a "housane"). Ring-opening ensues, followed by the shift of the blue CH2 group in one of two possible directions. An electron is then added back to the system to produce the two products shown. Interestingly, the product on the left is formed preferentially and the ratio of the two products appears to be insensitive to the electronic properties of X. While the Little group is examining these rearrangements in the lab, we are attempting to map out their mechanisms and uncover the factors that control their regio- and stereoselectivity using quantum chemical calculations.
Gerkin, J. B.; Wang, S. C.; Preciado, A. B.; Park, Y. S.; Nishiguchi, G.; Tantillo, D. J.; Little, R. D. J. Org. Chem. 2005, 70, 4598-4608: "Remote Substituent Effects upon the Rearrangements of Housane Cation Radicals"
Park, Y. S.; Wang, S. C.; Tantillo, D. J.; Little, R. D. J. Org. Chem., in press: "A Highly Selective Rearrangement of a Housane-derived Cation Radical; an Electrochemically Mediated Transformation"