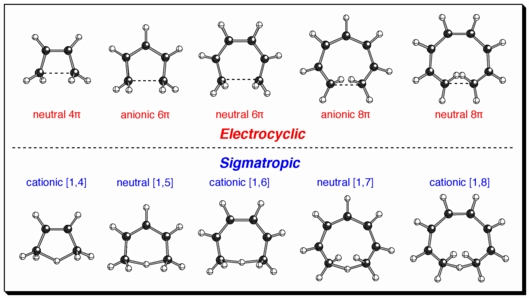
The principle of orbital symmetry conservation implies that the topology of the carbon backbones in transition structures for different types of pericyclic reactions will be similar. But it does not say how similar. We have shown that electrocyclic and sigmatropic rearrangements with the same electron count have transition state geometries that are strikingly similar, in fact almost indistinguishable (see above) but for the presence or absence of a simple proton (in yellow, below, in the cationic [1,8]-shift/neutral 8-pi cyclization example). These observations have also led to the concept of "transition state proton affinities", a measure of the basicity of partially formed or broken bonds in pericyclic transition structures. In addition, transition structures for sigmatropic reactions also have substructures that resemble 3-center 2-electron cations (in red, below).
We have examined various properties of transition state structures for various pericyclic reactions:
Hoffmann, R.; Tantillo, D. J. Angew. Chem. Int. Ed. 2003, 42, 5877-5882: "Breaking Down Barriers: The Liaison Between Sigmatropic Shifts, Electrocyclic Reactions and 3-Center Cations"
Ponec, R.; Bultinck, P.; Van Damme, S.; Carbo, R.; Tantillo, D. J. Theor. Chem. Acc. 2005, 113, 205-211: "Geometric and Electronic Similarities between Transition Structures for Electrocyclizations and Sigmatropic Hydrogen Shifts"
Tantillo, D. J. Annu. Rep. Prog. Chem., Sect. B 2006, 102, 269-289: "Reaction Mechanisms. Part (ii) Pericyclic Reactions"
Tantillo, D. J.; Lee, J. K. Annu. Rep. Prog. Chem., Sect. B 2007, 103, 272-293: "Reaction Mechanisms. Pericyclic Reactions"
Lee, J. K.; Tantillo, D. J. Annu. Rep. Prog. Chem., Sect. B 2008, 104, 260-283: "Reaction Mechanisms. Pericyclic Reactions"
Nouri, D. H.; Tantillo, D. J. Tetrahedron 2008, 64, 5672-5679: "Sigmatropic Shifts and Cycloadditions on Neutral, Cationic, and Anionic Pentadienyl + Butadiene Potential Energy Surfaces"
Tantillo, D. J. Angew. Chem. Int. Ed. 2009, 48, 31-32: "Using Theory and Experiment to Discover Catalysts for Electrocyclizations," invited Highlight.
Lodewyk, M. W.; Kurth, M. J.; Tantillo, D. J. J. Org. Chem. 2009, 74, 4804-4811: "Mechanisms for Formation of Diazocinones, Pyridazines, and Pyrazolines from Tetrazines - Oxyanion-Accelerated Pericyclic Cascades?"
Tantillo, D. J.; Lee, J. K. Annu. Rep. Prog. Chem., Sect. B 2009, 105, 285-309: "Reaction Mechanisms. Pericyclic Reactions"
Siebert, M. R.; Osbourn, J. M.; Brummond, K. M.; Tantillo, D. J. J. Am. Chem. Soc. 2010, 132, 11952-11966: "Differentiating Mechanistic Possibilities for the Thermal, Intramolecular [2 + 2] Cycloaddition of Allene-ynes"
Harrison, J. G.; Tantillo, D. J. Phys. Chem. Chem. Phys. 2012, 14, 14756-14759: "Fusing Cubanes to 1,5-Hexadiene," invited for themed issue on "Predicting New Molecules by Quantum Chemical Methods".
Maity, P.; Pemberton, R. P.; Tantillo, D. J.; Tambar, U. K. J. Am. Chem. Soc. 2013, 135, 16380-16383: "Bronsted Acid Catalyzed Enantioselective Indole Aza-Claisen Rearrangement Mediated by an Arene CH-O Interaction"
Painter, P. P.; Wong, B. M.; Tantillo, D. J. Org. Lett. 2014, 16, 4818-4821: "Facilitating the Cope Rearrangement by Partial Protonation - Implications for Synthesis and Biosynthesis"
Rasik, C. M.; Hong, Y. J.; Tantillo, D. J.; Brown, M. K. Org. Lett. 2014, 16, 5168-5171: "Origins of Diastereoselectivity in Lewis Acid-Promoted Ketene-Alkene [2+2] Cycloadditions"
Slauson, S. R.; Pemberton, R. P.; Ghosh, P.; Tantillo, D. J.; Aube, J. J. Org. Chem. 2015, 80, 260-5271: "Domino Acylation/Diels-Alder Synthesis of N-Alkyl-octahydroisoquinolin-1-one-8-carboxylic Acids Under Low-Solvent Conditions"
Larson, R. T.; Pemberton, R. P.; Franke, J. M.; Tantillo, D. J.; Thomson, R. J. J. Am. Chem. Soc. 2015, 137, 11197-11204: "Total Synthesis of the Galbulimima Alkaloids Himandravine and GB17 using Biomimetic Diels–Alder Reactions of Double Diene Precursors"
Painter, P. P.; Siebert, M. R.; Tantillo, D. J. J. Org. Chem., in press, DOI: 10.1021/acs.joc.5b00996: "Conjugate Addition/[3,3] Sigmatropic Shift Processes for Formation of Medium Ring Cyclic Amines - Do They Circumvent the Woodward-Hoffmann Rules?," part of special issue: "50 Years and Counting: The Woodward-Hoffmann Rules in the 21st Century"
Sharma, K.; Wolstenhulme, J. R.; Painter, P. P.; Yeo, D. Carmona, F. G.; Johnston, C. P.; Tantillo, D. J.; Smith, M. D. J. Am. Chem. Soc. 2015, 137, 13414-13424: "Cation-Controlled Enantio- and Diastereoselective Synthesis of Indolines: An Autoinductive Phase-Transfer Initiated 5-endo-trig Process"
Kocsis, L. S.; Kagalwala, H. N.; Mutto, S.; Godugu, B.; Bernhard, S.; Tantillo, D. J.; Brummond, K. M. J. Org. Chem. 2015, 80, 11686-11698: "Mechanistic Insight into the Dehydro-Diels-Alder Reaction of Styrene-ynes," part of special issue: "50 Years and Counting: The Woodward-Hoffmann Rules in the 21st Century"
Painter, P. P.; Siebert, M. R.; Tantillo, D. J. J. Org. Chem. 2015, 80, 11699-11705: "Conjugate Addition/[3,3] Sigmatropic Shift Processes for Formation of Medium Ring Cyclic Amines - Do They Circumvent the Woodward-Hoffmann Rules?," part of special issue: "50 Years and Counting: The Woodward-Hoffmann Rules in the 21st Century"
Tantillo, D. J., Acc. Chem. Res. 2016, 49, 741-749: "Speeding Up Sigmatropic Shifts - To Halve or to Hold," invited account for special issue on "Computational Catalysis for Organic Synthesis."
Nguyen, Q. N. N.; Tantillo, D. J. J. Org. Chem. 2016, 81, 5295-5302: "When to Let Go - Diradical Intermediates from Zwitterionic Transition State Structures?"
Hare, S. R.; Tantillo, D. J. J. Chem. Educ. 2017, 94, 988-993: "Pericyclic or Pseudopericyclic? The Case of an Allylic Transposition in the Synthesis of a Saccharin Derivative" (a "reply": DOI: 10.1021/acs.jchemed.7b00373)
Xu, Y.; Hong, Y. J.; Tantillo, D. J.; Brown, M. K. Org. Lett. 2017, 19, 3703-3706: "Intramolecular Chirality Transfer [2+2] Cycloadditions of Allenoates and Alkenes"
Zhu, J. S.; Son, J.-H.; Teuthorn, A. P.; Haddadin, M. J.; Kurth, M. J.; Tantillo, D. J. J. Org. Chem. 2017, 82, 10875-10882: "Diverting Reactive Intermediates Toward Unusual Chemistry - Unexpected Anthranil Products from Davis–Beirut Reaction" (correction to SI: DOI:10.1021/acs.joc.7b02695)
Dimirjian, C. A.; Castiñeira Reis. M.; Balmond, E. I.; Turman, N. C.; Rodriguez, E. P.; Di Mas, M. J.; Fettinger, J. C.; Tantillo, D. J.; Shaw, J. T. Org. Lett. 2019, 21, 7209-7212: "Synthesis of Spirobicyclic Pyrazoles by Intramolecular Dipolar Cycloadditions/[1s, 5s] Sigmatropic Rearrangements"
Rayo, D. F. L.; Hong, Y.J.; Campeau, D.; Tantillo, D. J.; Gagosz, F. Chem. Eur. J. 2021, 27, 10637-10648: "On the Mechanism of Au-catalyzed Enynamide-Yne Dehydro-Diels-Alder Reactions: An Experimental and Computational Study"
Hashimoto, Y.; Tantillo, D. J. J. Org. Chem. 2022, 87, 12954-12962: "Mechanism and the Origins of Periselectivity in Cycloaddition Reactions of Benzyne with Dienes"
Sanchez, A.; Gurajapu, A.; Guo, W.; Kong, W.-Y.; Laconsay, C. J.; Settineri, N. S.; Tantillo, D.J.; Maimone, T. J. J. Am. Chem. Soc. 2023, 145, 13452-13461: "A Shapeshifting Roadmap for Polycyclic Skeletal Evolution"

