
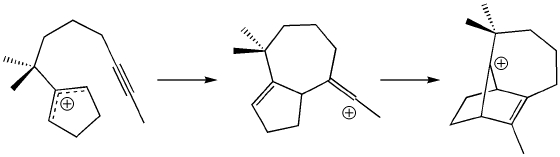
William S. Johnson's 1975 synthesis of longifolene was a triumph. Not only did it demonstrate the ever growing prowess of organic synthesis, but it also showcased the viability of enzyme-free carbocation polycyclizations. Moreover, it demonstrated that knowledge of and speculation on the biosynthesis of complex organic molecules can be a useful source of inspiration for synthesis design; this remains true today. Using the tools of modern quantum chemistry, we have examined the mechanism of the proposed allyl-to-alkenyl-to-norbornenyl polycyclization (shown above) used for forming the core of longifolene. Our calculations show that this process is indeed energetically viable and results in a nonclassical norbornenyl cation.
Ho, G. A.; Nouri, D. Tantillo, D. J. J. Org. Chem. 2005, 70, 5139-5143: "The Cationic Cascade Route to Longifolene"