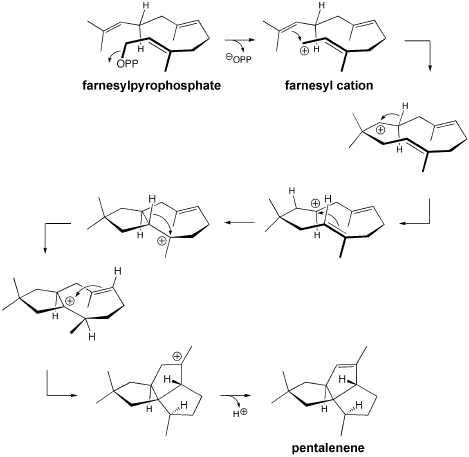
Polycyclization reactions are single transformations in which several new rings are formed. These sorts of reactions have been used by Nature, as well as organic chemists, to achieve rapid and efficient syntheses of complex polycycles. We are performing calculations on a variety of reactions belonging to a subset of polycyclization reactions that we refer to as Reactive Intermediate Promoted Polycyclization (RIPP) reactions. This class of reactions includes all polycyclization reactions that are initiated by the generation of an organic reactive intermediate. Our initial focus has been on cations and radicals, although anions, radical ions, etc. are also possible and represent additional areas into which our research may expand. One of the first RIPP reactions we chose to explore is shown above. This cation-promoted polycyclization was proposed as a mechanism involved in the biosynthesis of pentalenene, a complex terpenoid natural product. Another example is described here:
Wang, S. C.; Tantillo, D. J. Org. Biomol. Chem. 2017, 15, 1976-1979: "Viability of Dodecahedrane-Forming Radical Polycyclizations," contribution to themed issue on "Polycylizations in Synthesis and Biosynthesis," featured on the outside front cover.