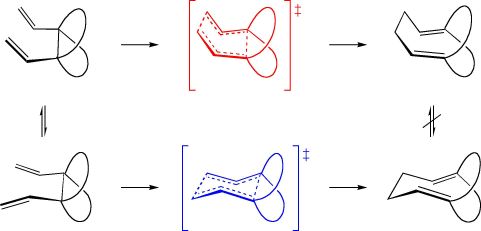
1,5-Hexadiene can undergo a Cope rearrangement ([3,3]-sigmatropic shift) through either a chairlike or boatlike transition state, but the chairlike pathway is preferred by approximately 11 kcal/mol. Using hybrid HF-DFT calculations, we showed that hexadienes in which the 3,4-carbon-carbon bond is fused to two hydrocarbon rings (above, left) rearrange through chairlike and boatlike transition states with nearly identical energies. We refer to such systems as "fickle hexadienes" because of their inability to choose between chairlike and boatlike pathways.
Tantillo, D. J.; Hoffmann, R. J. Org. Chem. 2002, 67, 1419-1426: "Fickle Hexadienes. Manipulating the Relative Energies of Chairlike and Boatlike Transition Structures for the Cope Rearrangement"