
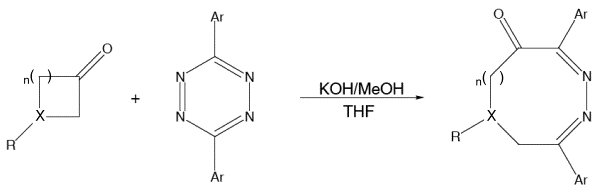
The synthesis of diazocinones via condensation of tetrazines with various enolates derived from cyclic ketones (above) was reported by Kurth and coworkers. We have carried out theoretical studies on these (and related) reactions, and our calculations have revealed two distinct mechanistic pathways by which they can occur: one involving a ring-opening step that is electrocyclic in nature and another involving a ring-opening step that is analogous to a retro-aldol reaction. Which of these pathways predominates, as well as whether or not the ring-opening step actually occurs, depends on the structural features present in the reactants. For example, the amount of ring strain present in the enolate appears to be directly related to the facility of the ring-opening step.
Lodewyk, M. W.; Kurth, M. J.; Tantillo, D. J. J. Org. Chem. 2009, 74, 4804-4811: "Mechanisms for Formation of Diazocinones, Pyridazines, and Pyrazolines from Tetrazines - Oxyanion-Accelerated Pericyclic Cascades?"
Ye, L.; Haddadin, M. J.; Lodewyk, M. W.; Ferreira, A.; Fettinger, J. C.; Tantillo, D. J.; Kurth, M. J. Org. Lett. 2010, 12, 164-167: "Cyclopenta[b]pyrroles from Triazines - Synthetic and Mechanistic Studies"
Michael W. Lodewyk and Dean J. Tantillo: "Mechanistic Studies on Diazocinone-Forming Cyclization." Poster presented by Mike Lodewyk at the 232nd ACS National Meeting, San Francisco, CA, September 10-14, 2006; paper ORGN 824.
Michael W. Lodewyk and Dean J. Tantillo: "Mechanistic Studies on Diazocinone-Forming Cyclization." Poster presented by Mike Lodewyk at the SYLICCO.07 Symposium, Davis, CA, July 26, 2007.