
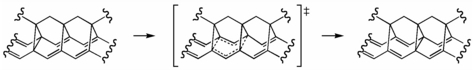
One of our initial proposals for a potential "[3,3] sigmatropic shiftamer" is shown above. We had hoped that polymers of this type would undergo degenerate Cope rearrangements ([3,3] sigmatropic shifts) with low activation barriers, making for fluxional polymers that could transport the electrons in a sigma-bond back-and-forth along their backbones of parallel pi-bonds.
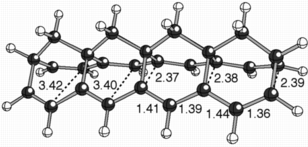
When we explored discrete model systems for this polymer, we discovered that, rather than structures with localized sigma-bonds connecting their two polyene chains, structures were formed that appeared to have two polyenyl radical chains interacting with each other through space (see the structure above for an example, selected distances are shown in angstroms).
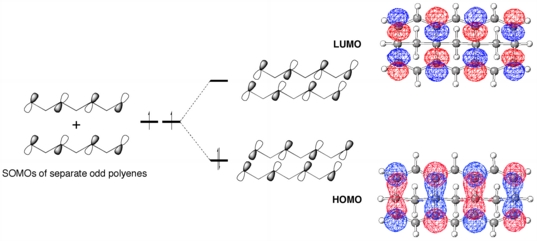
These structures are similar to a class of molecules called "polyacenes" - ribbons of fused aromatic rings like anthracene, tetracene, etc. - in that both involve the interaction of polyenyl chains. In the polyacenes, the polyeneyl orbitals interact in a pi-sense, while in the compounds we happened upon (which we now call "sigma-polyacenes"), the polyenyl orbitals interact face-to-face, in a sigma-sense, as shown above (orbital cartoons are shown at the left and the actual HOMO and LUMO from our calculations are shown at the right).
Tantillo, D. J.; Hoffmann, R.; Houk, K. N.; Warner, P. M.; Brown, E. C.; Henze, D. K. J. Am. Chem. Soc. 2004, 126, 4256-4263: "Extended Barbaralanes - Sigmatropic Shiftamers or sigma-Polyacenes?"