
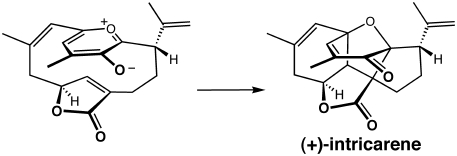
We have used quantum chemical calculations to explore the mechanism, regio-, and stereoselectivity of oxidopyrylium–alkene (and related) cycloadditions, including those used in two reported total syntheses of the diterpenoid (+)-intricarene, and possibly also occurring during its biosynthesis. For intricarene, we addressed: (1) the necessity of enzyme intervention if this reaction occurs in Nature, and (2) the effects of substituents attached to the oxidopyrylium and alkene groups on the activation barriers for cycloaddition.
Wang, S. C.; Tantillo, D. J. J. Org. Chem. 2008, 73, 1516-1523: "Theoretical Studies on Synthetic and Biosynthetic Oxidopyrylium–Alkene Cycloadditions. Pericyclic Pathways to Intricarene"
Hare, S. R.; Farnham, J. M.; Tantillo, D. J. Tetrahedron 2017, 73, 4227-4232: "Putative Biosynthetic Cycloadditions en route to the Diterpenoid (+)-Chatancin"
Kaufman, R. H.; Law, M. C.; Simanis, J. A.; Woodall, E. L.; Zwick, C. R., III; Wedler, H. B.; Wendelboe, P.; Hamaker, C. G.; Goodell, J. R.; Tantillo, D. J.; Mitchell, T. A. J. Org. Chem. 2018, 83, 9818-9838: "Oxidopyrylium-Alkene [5 + 2] Cycloaddition Conjugate Addition Cascade (C3) Sequences: Scope, Limitation, and Computational Investigations"
Lu, Y.; Tantillo, D. J. J. Org. Chem. 2021, 86, 8652-865: "Comparison of (5+2) Cycloadditions Involving Oxidopyrylium and Oxidopyridium Ions – Relative Reactivities"
Corrie, S. I.; Pearce, A. C.; Grabowski, J. P.; Randolph. K.; Guo, W.; Tantillo, D. J.; Mitchell, T. A. Tetrahedron Lett. 2022, 107, 154094: "Boron-tethered Oxidopyrylium-based [5+2] Cycloadditions"
Youman, A. J.; Rokey, S. N.; Grabowski, J. P.; Guo, W.; Sun, Q.; Angles, S.; Goodell, J. R.; Tantillo, D. J.; Mitchell, T. A. J. Org. Chem. 2023, 88, 5972-5981: "Experimental and Theoretical Investigation of the Synchronicity of Ambident Silyloxypyrone-based (5 + 2) Cycloadditions"
Angles, S. N.; Guo, W.; Darko, K.; Erzuah, M.; Pauley, K.; Promise, I. E.; Goodell, J. R.; Tantillo, D. J.; Mitchell, T. A. Org. Lett. 2023, 25, 7137-7141: "Net Intermolecular Silyloxypyrone-based (5 + 2) Cycloadditions Utilizing Amides as Enabling and Cleavable Tethers"