
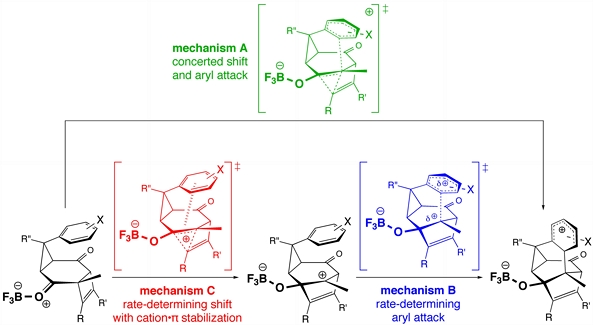
Quantum mechanical calculations were used to study the substituent effects and the concertedness of the alkenyl migration/electrophilic aromatic substitution reactions recently reported by Oshima and coworkers (above). Our calculations suggest that these systems prefer stepwise mechanisms with their aryl attack steps having the highest energy transition structures (blue scenario above), but that in some highly substituted cases, effectively concerted but very asynchronous processes may occur (green scenario above).
Wang, S. C.; Tantillo, D. J. J. Org. Chem. 2007, 72, 8394-8401: "Substituent Effects on Tandem Alkenyl Migration/Electrophilic Aromatic Substitution Reactions"
Kazim, M.; Feng, Z.; Vemulapalli, S.; Siegler, M. A.; Chopra, A.; Nguyen, P. M.; Holl, M. G.; Guan, L.; Dudding, T.; Tantillo, D.J.; Lectka, T. Chem. Eur. J. 2023, e202301550: "Through-Space, Lone-Pair Promoted Aromatic Substitution: A Relay Mechanism Can Beat Out Direct Activation"