
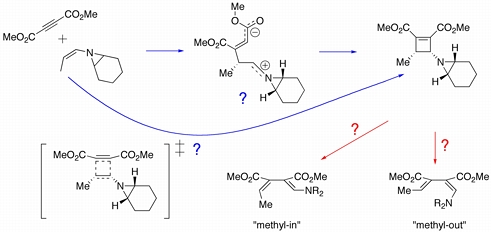
The cycloaddition of (Z)-7-(prop-1-enyl)-7-azabicyclo[4.1.0]heptane with dimethyl acetylene dicarboxylate was reported by Yudin and coworkers to proceed with complete stereoselectivity. Quantum chemical calculations were used to evaluate the mechanism of the cyclization process, and it was discovered that a stepwise pathway (through the zwitterion shown above) is preferred. The subsequent electrocyclic ring opening reaction of the resulting cyclobutene was also studied and it was found that ring-opening to the "methyl-in" dienamine is preferred to the "methyl-out" product by some 4-5 kcal/mol.
We have also examined a variety of other reactions of aziridines.
Siebert, M. R.; Yudin, A. K.; Tantillo, D. J. Org. Lett. 2008, 10, 56-60: "Cycloaddition/Ring Opening Reaction Sequences of N-Alkenyl Aziridines: Influence of the Aziridine Nitrogen on Stereoselectivity"
Siebert, M. R.; Yudin, A. K.; Tantillo, D. J. Eur. J. Org. Chem. 2011, 553-561: "The Effect of Strain on the Rh(I)-Catalyzed Rearrangement of Allyl Amines"
Siebert, M. R.; Tantillo, D. J. J. Phys. Org. Chem. 2011, 24, 445-449: "Fundamental Properties of N-Alkenylaziridines - Implications for the Design of New Reactions and Organocatalysts"