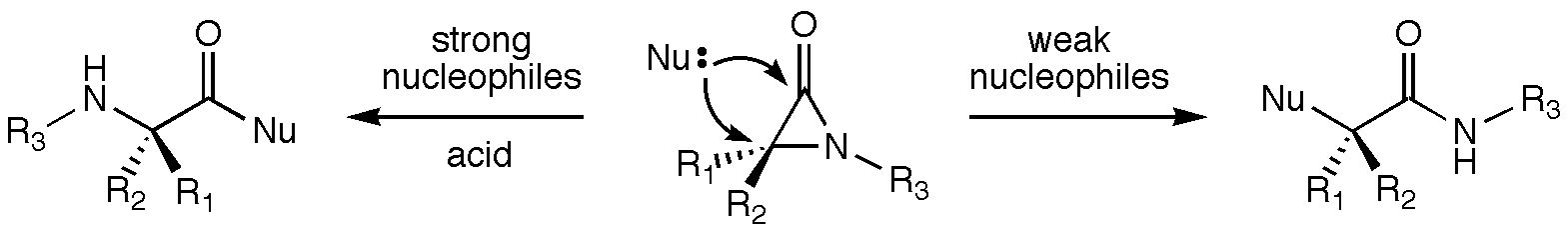
alpha-Lactams (aziridinones) have been shown by Robert Hoffman in the Department of Chemistry and Biochemistry at New Mexico State University (and others) to react with nucleophiles at both the acyl (C-2) and a (C-3) carbons. Products of C-2 attack are observed with strong nucleophiles, and products of C-3 attack are observed with weak nucleophiles in the presence of Bronsted and Lewis acids. C-3 attack proceeds with inversion of configuration at the C-3 carbon. A cationic intermediate activated towards C-3 attack has been implicated in this substitution process, but the structure of this intermediate is unknown. Density functional theory calculations were used to examine various candidate structures for this intermediate and to assess their reactivity. Initial protonation on oxygen or nitrogen leads to several cyclic cations with relative energies within several kcal/mol of each other. Direct nucleophilic attack at both C-2 and C-3 of these structures was examined, and the substitution transition state with the lowest relative energy corresponds to C-3 attack on the O-protonated species with inversion of configuration at C-3. Involvement of this process is consistent with the experimental results.
Tantillo, D. J.; Houk, K. N.; Hoffman, R. V.; Tao, J. J. Org. Chem. 1999, 64, 3830-3837: "The Origins of Regio- and Stereoselectivity in Acid-Promoted Reactions of alpha-Lactams"